|
||
|
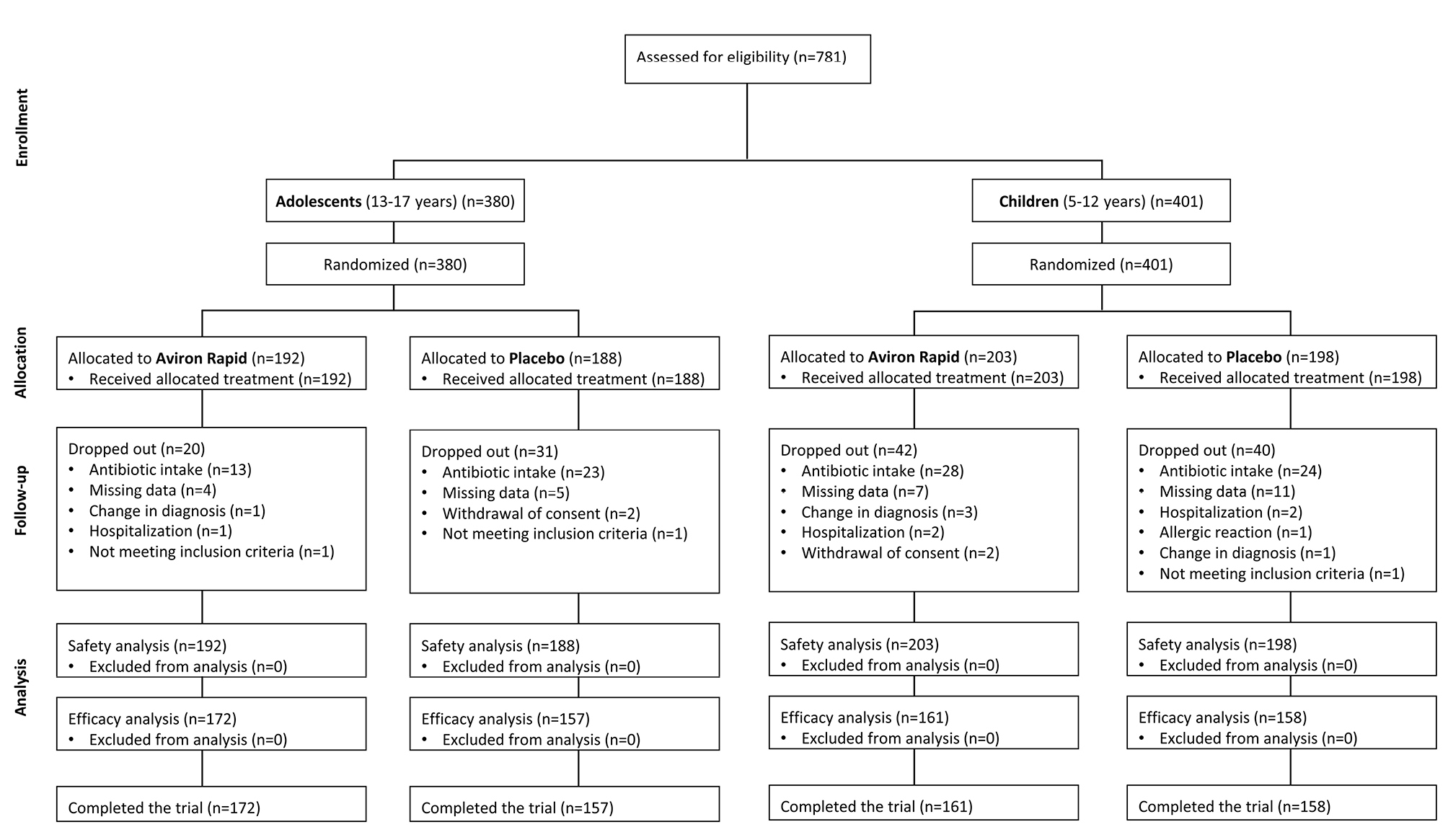
Patient disposition (Safety Analysis). Safety analysis included all patients who received at least one dose of Aviron Rapid or placebo. Efficacy analysis included all patients who completed treatment and all planned trial visits per protocol, and did not have protocol deviations. |